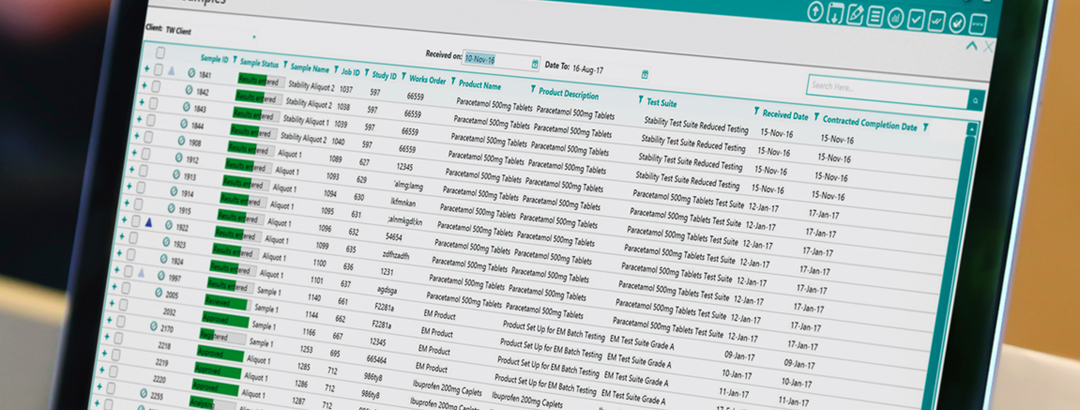
Advancements in technology have allowed the availability and delivery of software to be quicker, more secure and more reliable. If you’re still using on-premise LIMS, here are a few reasons why you should consider switching to SaaS LIMS.
SaaS vs On-premise – Why Now is the Time to Consider a SaaS LIMS
by Sophie Blackburn posted in LIMS, Data Management, Process Improvement, best practices, process mapping, deployment projects, Laboratory Efficiency, Laboratory Management, Tips4 Reasons Why You Don't Have LIMS and Tips to Overcome Them
by Sophie Blackburn posted in LIMS, Data Management, Process Improvement, best practices, process mapping, deployment projects, Laboratory Efficiency, Laboratory Management, TipsLaboratory information management systems (LIMS) are known to deliver substantial efficiencies to laboratory operations. From sample management, data comparison and reporting, LIMS can transform the most time-consuming, mundane and critical tasks to simple, easy-to-complete tasks within a click of a button. So, why doesn’t every lab have a LIMS?
LabHQ's Best Features for Pharma
by Sophie Blackburn posted in LIMS, Data Management, Process Improvement, best practices, process mapping, deployment projects, Laboratory Efficiency, Laboratory Management, TipsThe pharmaceutical industry is highly regulated to ensure that the quality, efficacy and safety of medicines delivered to patients is maintained. When pharma companies
What to Do When Your LIMS is No Longer Supported
by Theresa Webster posted in LIMS, Data Management, Process Improvement, best practices, process mapping, deployment projects, Laboratory Efficiency, Laboratory Management, TipsAs a laboratory that has already made the leap from paper to LIMS, moving away from LIMS would be a step backwards, and certainly wouldn’t be the favourable option if your LIMS is no longer supported. What do you do when your LIMS supplier has discontinued service? In this blog, we go through the key areas to consider, so you don’t have to roll back to paper-based systems.
Are Risk Assessments Required for a LIMS Project?
by Theresa Webster posted in Project Management, Process Improvement, Laboratory Efficiency, Laboratory Management, Laboratory Team, Tips, Workload ManagementHave you ever taken on a project that is large and overwhelming that you didn’t know where to start? It can feel like everything is blurry, and you’re looking for your glasses to bring your sight into focus. Deploying a LIMS can often feel like this, and risk assessments can help you bring the project to focus.
Have You Seen the Latest Update of ISO/IEC 17025?
by Phil Saunders posted in LIMS, Data Management, Project Management, Process Improvement, QC Laboratory Data Management, Quality, Laboratory Efficiency, Laboratory Management, Laboratory Team, Tips, Workload ManagementLaboratories look to gain ISO 17025 accreditation as recognition of their competence and abilities, to help them improve and monitor their operational processes, and to satisfy customer requirements. At the end of 2017, an update to the ISO 17025 standard was issued. In this blog, we give you
How to Balance Your Time Between Routine Operations and Improvement Projects
by Theresa Webster posted in Project Management, Process Improvement, Laboratory Efficiency, Laboratory Management, Laboratory Team, Tips, Workload ManagementLaboratory Managers are frequently overwhelmed with the amount of work they need to complete. Especially in a Quality Control laboratory, where turnaround times are crucial, routine operations continually takes priority, but what if
Is Your LIMS Good Enough? Where LIMS Adds the Most Value for Labs
by Theresa Webster posted in LIMS, Data Management, Data Integrity, Process Improvement, process mapping, QC Laboratory Data Management, Quality
When you’re working in a fast-paced environment such as a laboratory, you might not have the time to implement improved ways of working. You may be using an existing LIMS, and are used to the workarounds and quirks of the system.
I Know I Need LIMS But What Do I Do Now?
by Phil Saunders posted in LIMS, Data Management, Project Management, Process Improvement, LabHQ, process mapping, QC Laboratory Data Management, Quality, deployment projects
4 Signs Your Lab Has Outgrown Your LIMS
by Theresa Webster posted in LIMS, Data Management, Data Integrity, Process Improvement, process mapping, QC Laboratory Data Management, QualityMany laboratories introduce LIMS to help with sample data management and reporting. Some labs will have used the same system for 12 years without upgrades, and other labs will